Ishikawa Fishbone Root Cause Analysis
Introduction to Fishbone Analysis
Fishbone Analysis, also known as Ishikawa analysis or cause-and-effect diagram, is a structured tool used to identify the root causes of a specific problem by dissecting it into contributing factors. The method, created by Japanese quality management expert Kaoru Ishikawa, visually represents the problem and its causes in a "fishbone" diagram, helping teams systematically analyze all the possible sources of an issue.
In the pharmaceutical and biopharmaceutical industries, where stringent quality control and risk management practices are paramount, Fishbone Analysis becomes a critical tool. These industries are characterized by complex production processes, regulatory requirements, and high-stakes consequences for failure. Whether addressing manufacturing inefficiencies, product recalls, or deviations in production, Fishbone Analysis offers a thorough and collaborative approach to solving problems.
This article will explore how Fishbone Analysis can be applied within the pharmaceutical and biopharma sectors to enhance processes, ensure compliance, and safeguard product quality. The content is divided into the following sections:
- Overview of Fishbone Analysis
- Importance in Pharma & Biopharma
- Applications in Key Areas
- Quality control and assurance
- Manufacturing
- Research and Development (R&D)
- Regulatory compliance
- Steps for Implementing Fishbone Analysis
- Case Studies
- Challenges and Best Practices
1. Overview of Fishbone Analysis
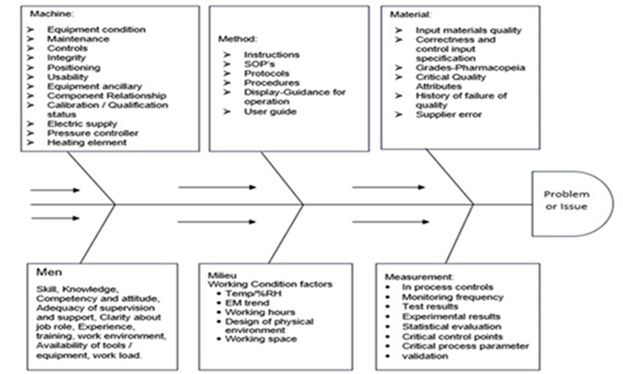
The Fishbone Analysis diagram is a graphical representation used to brainstorm and explore possible causes of a particular problem or effect. The diagram resembles the skeleton of a fish, where the "head" represents the problem, and the "bones" extending outward symbolize the categories of potential causes. The method involves dissecting the problem into major categories, such as people, methods, machinery, materials, environment, and measurement (commonly referred to as the 6Ms), although this can be adapted depending on the industry.
Each of these categories is further broken down into sub-causes, revealing deeper insights into the roots of the issue. In pharmaceutical and biopharmaceutical contexts, these categories can be modified to reflect industry-specific concerns, such as regulatory compliance, supply chain issues, or technological constraints.
The main objective is to:
- Identify all potential causes of a problem.
- Organize and prioritize the causes for detailed investigation.
- Provide a framework for cross-functional collaboration, leading to well-rounded solutions.
2. Importance of Fishbone Analysis in Pharma & Biopharma
The pharmaceutical and biopharmaceutical industries operate under strict regulatory guidelines enforced by organizations like the FDA, EMA, and WHO. Quality issues, safety concerns, or process deviations can have significant consequences, from product recalls to loss of market authorization and, more importantly, risks to patient safety.
In these highly regulated sectors, the importance of root cause analysis (RCA) and continuous improvement cannot be overstated. Fishbone Analysis becomes a valuable asset in managing these risks because it offers:
- A structured approach to identifying causes of process inefficiencies, quality deviations, or compliance gaps.
- A holistic view of the problem by considering technical, procedural, human, and environmental factors.
- Cross-functional collaboration, encouraging input from various departments such as manufacturing, quality assurance, regulatory affairs, and R&D, fostering better problem-solving.
- Documentation for regulatory audits, as it helps companies provide detailed reports on root cause investigations and corrective actions.
3. Applications in Key Areas of Pharma & Biopharma
Fishbone Analysis can be used in various critical areas within the pharmaceutical and biopharma sectors:
A. Quality Control & Assurance
- Product Recalls & Deviation Investigations: When a product recall or deviation occurs, Fishbone Analysis helps companies systematically investigate causes such as contamination, equipment malfunction, human error, or inadequate process controls.
- Out-of-Specification (OOS) Results: In cases where test results fall outside the predefined specification limits, the Fishbone diagram assists in identifying whether issues arise from lab procedures, reagent quality, analyst errors, or equipment malfunctions.
- Corrective and Preventive Actions (CAPA): Fishbone Analysis helps in designing robust CAPA plans by identifying the root causes of quality failures or deviations and ensuring that preventive measures address all contributing factors.
B. Manufacturing
- Batch Failures & Yield Loss: For pharmaceutical manufacturers, production inefficiencies or batch failures can be extremely costly. Fishbone Analysis aids in isolating causes such as equipment calibration, operator training, raw material variability, or environmental factors like temperature and humidity control.
- Sterility Failures in Biopharma: In biopharmaceuticals, where sterility and contamination control are crucial, Fishbone Analysis is often used to address sterility failures by exploring all potential sources, including equipment cleaning protocols, operator handling, and facility design.
- Equipment Malfunctions: Manufacturing equipment breakdowns or suboptimal performance can lead to production delays and quality issues. By using Fishbone Analysis, teams can pinpoint causes like wear and tear, incorrect calibration, or lack of preventive maintenance.
C. Research and Development (R&D)
- Process Development: In pharmaceutical R&D, developing stable and scalable manufacturing processes is essential. Fishbone Analysis is used to optimize processes by investigating variables such as reagent quality, process parameters, and equipment suitability.
- Clinical Trial Failures: Clinical trials are a high-cost phase of drug development, and failure at this stage can set projects back significantly. Fishbone Analysis assists in identifying trial failures due to patient recruitment issues, protocol deviations, data collection errors, or ineffective blinding procedures.
D. Regulatory Compliance
- Audit Readiness: In the pharmaceutical and biopharma sectors, audit failures can lead to major business disruptions. Fishbone Analysis can help companies prepare for regulatory audits by investigating potential causes of non-compliance, whether they stem from documentation gaps, improper training, or equipment validation issues.
- Supply Chain Disruptions: Regulatory expectations for traceability and quality across the supply chain are high. Fishbone Analysis is useful in addressing supply chain disruptions by identifying root causes, such as vendor quality issues, transportation delays, or customs clearance problems.
4. Steps for Implementing Fishbone Analysis
The implementation of Fishbone Analysis in pharma or biopharma requires a structured, step-by-step approach to ensure thorough investigation and actionable results.
Step 1: Define the Problem
The first step is to clearly articulate the problem or effect being investigated. It is essential to be specific. For example, a problem might be "Contamination observed in Batch X" or "Product stability failed during storage."
Step 2: Identify the Main Categories
The next step involves identifying the main categories of causes. Commonly, the 6Ms (Manpower, Materials, Methods, Machines, Measurement, and Environment) are used, but in pharma and biopharma, additional or modified categories might include:
- Regulations (e.g., compliance gaps)
- Technology (e.g., laboratory equipment issues)
- Supply Chain (e.g., raw material sourcing problems)
Step 3: Brainstorm Possible Causes
Under each category, brainstorm possible contributing factors. For example:
- Under Manpower, explore issues like lack of training, poor communication, or staff fatigue.
- Under Machines, consider equipment calibration, maintenance records, or operational settings.
Step 4: Analyze the Diagram
Once all potential causes have been identified, the next step is to analyze them for their likelihood and impact on the problem. Prioritize causes that are more likely to have contributed to the issue, and investigate these further through additional data collection or testing.
Step 5: Implement Corrective Actions
Based on the findings from the Fishbone Analysis, develop and implement corrective actions that address the root causes. These actions may involve changing processes, improving training, upgrading equipment, or enhancing quality control measures.
Step 6: Monitor and Review
After corrective actions are implemented, continuous monitoring is required to ensure that the problem does not recur. Regular reviews of the Fishbone diagram and associated causes should be conducted as part of a continuous improvement program.
5. Case Studies
Case Study 1: Investigating Contamination in Biopharma Manufacturing
A biopharmaceutical company faced repeated contamination in its sterile product line. Using Fishbone Analysis, the team identified potential causes under the following categories:
- Environment: Inadequate cleanroom controls and airflow patterns.
- Manpower: Inconsistent adherence to gowning procedures.
- Machines: Faulty sealing equipment for sterile containers.
Corrective actions included retraining staff, redesigning gowning procedures, and repairing equipment, ultimately leading to improved contamination control.
Case Study 2: Out-of-Specification (OOS) Results in Pharmaceutical Testing
A pharmaceutical quality control lab repeatedly experienced OOS results in drug potency testing. Through Fishbone Analysis, the team uncovered issues with:
- Materials: Degraded reagents used in testing.
- Methods: Outdated standard operating procedures (SOPs).
- Machines: Inconsistent calibration of the High-Performance Liquid Chromatography (HPLC) system.
The company corrected the SOPs, ensured regular calibration, and improved reagent storage, reducing OOS occurrences.
6. Challenges and Best Practices
Challenges
- Complexity in Pharma/Biopharma Settings: The intricate nature of pharmaceutical manufacturing and biopharma processes can make it difficult to accurately identify all potential causes.
- Data Availability: Lack of data or poor data quality can hinder effective analysis, leading to inaccurate conclusions.
- Cross-Departmental Coordination: Effective Fishbone Analysis requires collaboration across different functions, and miscommunication or lack of cooperation can slow down the investigation.
Best Practices
- Involve Cross-Functional Teams: To get a complete picture of the problem, involve experts from R&D, manufacturing, quality control, and regulatory affairs.
- Document Thoroughly: Maintain detailed records of the Fishbone Analysis process to provide traceability for audits and future investigations.
- Continuous Review: Fishbone Analysis should be part of a broader continuous improvement program, with regular reviews to ensure that identified causes are effectively mitigated.
Conclusion
Fishbone Analysis is an essential tool for root cause analysis and continuous improvement in the pharmaceutical and biopharmaceutical industries. It provides a structured, visual method for dissecting complex problems, helping companies ensure product quality, regulatory compliance, and manufacturing efficiency. By applying Fishbone Analysis across various functions, from quality control to manufacturing and R&D, organizations can identify and address the underlying causes of critical issues, ultimately leading to better products, safer processes, and higher customer satisfaction.
