Software Development Life Cycle (Sdlc); Computer System Validation (Csv)
Introduction to Computer System Validation (CSV)
Computer System Validation (CSV) is a critical process in regulated industries like pharmaceuticals, biotechnology, and healthcare, where adherence to stringent regulations ensures that software systems perform their intended functions accurately and consistently. The Good Automated Manufacturing Practice (GAMP) guidelines, particularly GAMP 5, provide a framework for ensuring that computer systems are developed and validated in a manner that is compliant with these regulations.
GAMP 5, introduced by the International Society for Pharmaceutical Engineering (ISPE), emphasizes a risk-based approach to compliance, aligning validation efforts with the complexity and risk associated with the system. The Software Development Life Cycle (SDLC) plays a crucial role in CSV by providing a structured approach to software development, ensuring that validation is integrated throughout the process.
This document explores the SDLC phases in the context of GAMP 5, detailing how each phase contributes to the overall validation of computer systems in regulated environments.
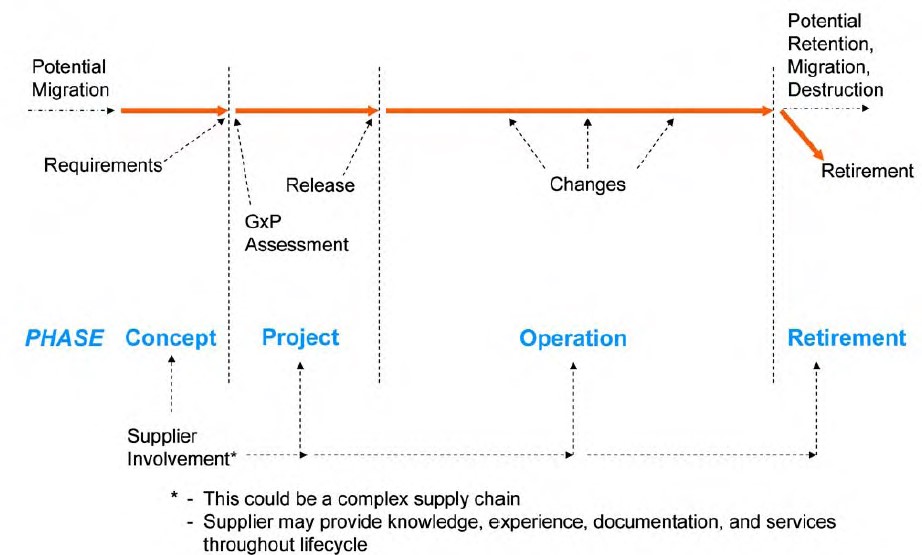
SDLC Phases in GAMP 5
The Software Development Life Cycle (SDLC) consists of several distinct phases, each contributing to the development and validation of a computer system. GAMP 5 outlines a set of best practices for each phase to ensure that the final product meets regulatory requirements and operates as intended.
1. Concept Phase
The Concept Phase is the foundation of the SDLC, where the initial idea for the software system is conceived. In GAMP 5, this phase involves defining the scope, objectives, and high-level requirements of the system. Key activities in this phase include:
- User Requirements Specification (URS): The URS is a critical document that outlines what the users expect from the system. It should include functional, non-functional, and regulatory requirements. In GAMP 5, the URS serves as a baseline for subsequent validation activities.
- Feasibility Study: A feasibility study assesses whether the proposed system can be developed and implemented within the constraints of time, budget, and technology. This study should consider the system's complexity, the regulatory environment, and the organization's capabilities.
- Risk Assessment: Early risk assessment identifies potential risks associated with the system and helps in defining the validation strategy. GAMP 5 advocates for a risk-based approach, focusing validation efforts on critical areas that impact patient safety, product quality, and data integrity.
2. Project Planning Phase
The Project Planning Phase is where detailed plans are created for the development and validation of the system. This phase sets the stage for successful project execution and ensures that all stakeholders are aligned with the project's goals.
- Project Plan: The project plan outlines the overall strategy for developing and validating the system. It should include timelines, resource allocation, roles and responsibilities, and a communication plan. The project plan must be aligned with GAMP 5 principles to ensure compliance.
- Validation Plan: The Validation Plan (VP) is a key document that describes how the validation activities will be carried out throughout the SDLC. It should include the scope of validation, acceptance criteria, and a detailed schedule of validation tasks.
- Risk Management Plan: Building on the initial risk assessment, the risk management plan provides a framework for ongoing risk management activities throughout the project. This plan should detail how risks will be monitored, mitigated, and communicated.
3. Requirements Specification Phase
The Requirements Specification Phase involves refining the requirements defined in the Concept Phase into detailed specifications that guide the system's design and development.
- Functional Requirements Specification (FRS): The FRS details the specific functions that the system must perform. It should be comprehensive, covering all aspects of the system's functionality, and traceable to the URS.
- Design Specification (DS): The DS translates the FRS into a detailed design that will guide the development of the system. It should include hardware and software architecture, data flow diagrams, and user interface designs. GAMP 5 emphasizes the importance of traceability, ensuring that every design element can be traced back to a user or functional requirement.
- Configuration Specification (CS): For configurable systems, the CS outlines the specific settings and parameters that need to be configured to meet the requirements. This document is particularly important for systems where customization is required to meet regulatory needs.
- Risk Assessment (Update): At this stage, the risk assessment is revisited to ensure that the detailed requirements and design do not introduce new risks. Any new risks identified should be documented, and mitigation strategies should be developed.
4. Design and Development Phase
The Design and Development Phase is where the system is built based on the specifications created in the previous phase. In GAMP 5, this phase emphasizes the importance of quality assurance and testing to ensure that the system meets the defined requirements.
- Development: During development, the system's software and hardware components are created according to the design specifications. This phase involves coding, configuration, and integration of various system components. GAMP 5 recommends that development activities adhere to industry best practices, such as coding standards, version control, and documentation.
- Unit Testing: Unit testing involves testing individual components or modules of the system to ensure they function correctly. GAMP 5 emphasizes the need for rigorous testing to identify and resolve defects early in the development process.
- Traceability Matrix: A traceability matrix is created to map requirements to design elements and test cases. This ensures that all requirements are covered and validated, and that any changes to the system are traceable to their source.
- Risk Assessment (Update): The risk assessment is updated to reflect any changes or new risks identified during the design and development phase. This ongoing risk management is a key aspect of GAMP 5's risk-based approach.
5. Testing and Verification Phase
The Testing and Verification Phase is critical in ensuring that the system meets all specified requirements and performs as intended. GAMP 5 provides guidance on different levels of testing and verification to ensure comprehensive validation.
- Integration Testing: Integration testing focuses on ensuring that different components or modules of the system work together as expected. This phase identifies issues that may arise from the interaction between components.
- System Testing: System testing involves testing the complete system in an environment that closely resembles the production environment. This testing verifies that the system meets all functional, non-functional, and regulatory requirements.
- User Acceptance Testing (UAT): UAT is conducted by the end-users to ensure that the system meets their needs and operates as expected in real-world scenarios. GAMP 5 emphasizes the importance of UAT in validating the system from the user's perspective.
- Performance Qualification (PQ): PQ testing verifies that the system performs consistently under expected operational conditions. This phase is particularly important in regulated industries where system performance can impact product quality or patient safety.
- Risk Assessment (Final Update): The risk assessment is finalized during this phase, ensuring that all identified risks have been mitigated or accepted. The final risk assessment report is a key deliverable that documents the system's risk profile at the time of validation.
6. Deployment Phase
The Deployment Phase involves the installation, configuration, and release of the system into the production environment. GAMP 5 emphasizes the importance of a controlled and documented deployment process to ensure that the system is correctly implemented and ready for use.
- Installation Qualification (IQ): IQ verifies that the system and its components are installed correctly according to the specifications. This includes checking hardware installation, software configuration, and network setup.
- Operational Qualification (OQ): OQ ensures that the system operates according to the design specifications under normal conditions. This phase involves executing test cases that simulate typical system operations.
- Go-Live Preparation: Before the system goes live, a series of readiness checks are performed to ensure that all aspects of the system are ready for production use. This includes finalizing user training, data migration, and backup procedures.
- Go-Live: The system is released into the production environment, and it begins its operational life. GAMP 5 recommends a closely monitored go-live process, with contingencies in place for any issues that may arise.
7. Operation and Maintenance Phase
The Operation and Maintenance Phase covers the entire period during which the system is in use. GAMP 5 stresses the importance of ongoing monitoring and maintenance to ensure that the system continues to operate correctly and remains compliant with regulatory requirements.
- System Monitoring: Continuous monitoring of the system's performance, security, and reliability is essential. GAMP 5 recommends implementing automated monitoring tools and regular audits to detect and address any issues promptly.
- Change Control: Any changes to the system, whether they are updates, patches, or enhancements, must be managed through a formal change control process. This ensures that all changes are documented, tested, and validated before being implemented in the production environment.
- Periodic Review: Regular reviews of the system are conducted to assess its ongoing compliance with regulatory requirements and its alignment with business needs. These reviews should be documented and any necessary corrective actions should be implemented.
- Incident Management: A process for managing incidents, such as system failures or security breaches, should be in place. GAMP 5 emphasizes the importance of documenting incidents, analyzing their root causes, and implementing corrective actions.
8. Retirement Phase
The Retirement Phase marks the end of the system's operational life. This phase involves decommissioning the system in a controlled and documented manner to ensure that all data is securely archived and that the system is safely removed from the production environment.
- Decommissioning Plan: A detailed plan for decommissioning the system is created, outlining the steps for safely shutting down the system, archiving data, and disposing of hardware and software components.
- Data Archiving: Before the system is decommissioned, all critical data must be securely archived in compliance with regulatory requirements. This ensures that data is preserved and can be accessed if needed in the future.
- Final Validation Report: A final validation report is created to document the decommissioning process and to confirm that all regulatory requirements have been met. This report is a key deliverable that serves as a record of the system's retirement.
- System Disposal: The physical and logical components of the system are safely disposed of, ensuring that no sensitive data is left behind. GAMP 5 recommends following industry best practices for secure disposal.
Conclusion
The Software Development Life Cycle (SDLC) is a critical framework for developing and validating computer systems in regulated industries. GAMP 5 provides a comprehensive set of guidelines for each phase of the SDLC, ensuring that computer systems are developed in a manner that is compliant with regulatory requirements and aligned with business needs.
By following the GAMP 5 guidelines throughout the SDLC, organizations can ensure that their computer systems are reliable, secure, and capable of consistently delivering high-quality products and services. This risk-based approach not only helps in meeting regulatory requirements but also enhances the overall quality and performance of the system.
The integration of validation activities into each phase of the SDLC, as outlined in GAMP 5, is essential for achieving and maintaining compliance in today's highly regulated environments. By adhering to these best practices, organizations can effectively manage the complexities of computer system validation and ensure the successful deployment of systems that are critical to their operations.
Image Credit & Reference: GAMP5
Visit our Service:https://likeways.co.in/resume-build-and-review/
