Good Documentation Practices (Gdps) And Alcoa Plus Principles For Data Integrity In Pharmaceutical
Good documentation practices that include entries and corrections to documents, responsibilities, and training requirements and also good documentation practices apply to both paper and electronic data or records filled manually or generated electronically in a GxP environment. It helps to understand who, when, where, why, and how to complete the relevant activities and provides evidence to show whether these have been completed as expected.
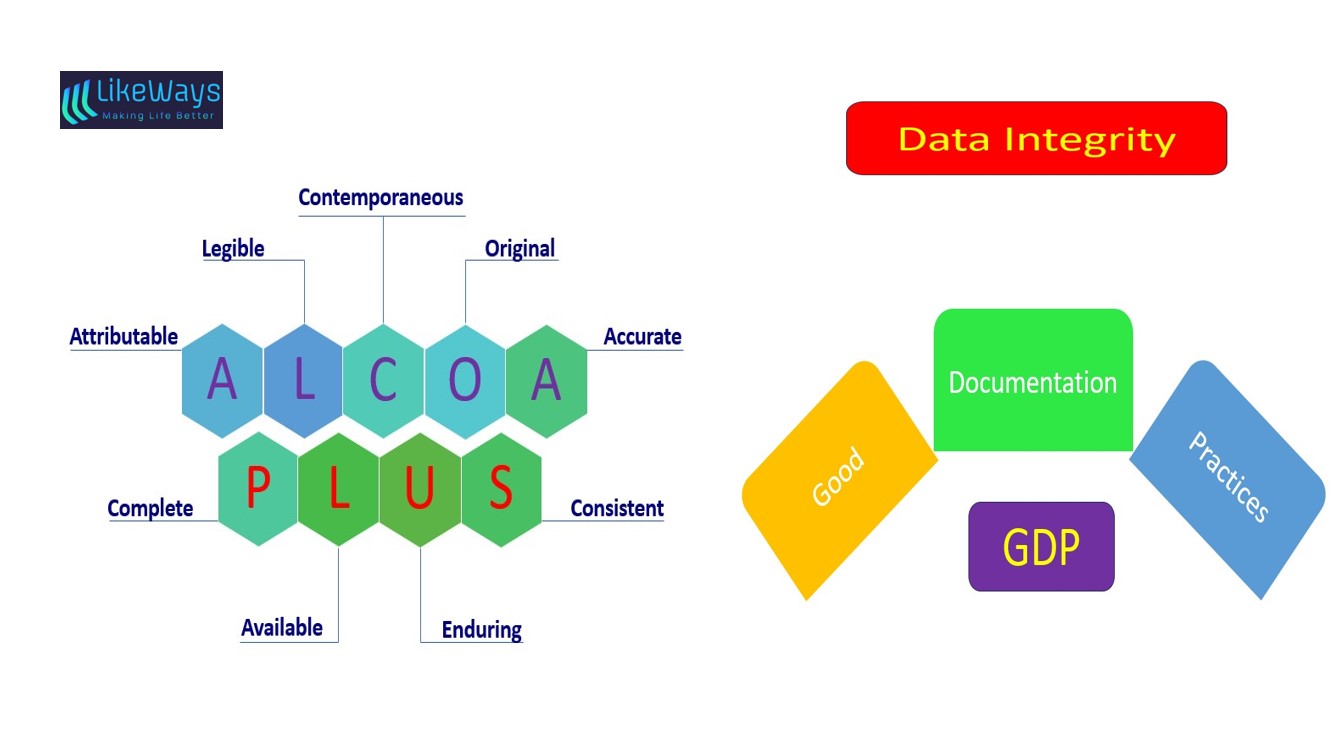
ALCOA is short for the essential characteristics of good documentation – Attributable, Legible, Contemporaneous, Original, and Accurate.The ALCOA guidelines put down by good documentation guidelines are applied to all forms of documentation and evidence maintained such as paper, electronic, and hybrid.
Scope:
Good Documentation Practices outline the only acceptable way to make entries and corrections to the documents, product batch records, laboratory records, product manufacturing documents, and reports and it is applicable for both paper and electronic data or records filled manually or generated electronically in a GxP environment.
Responsibilities:
All employees who are involved in document entries and/or corrections are responsible for recording accurate information.
Definitions:
Document: This is the official record or report that supports and /or records the activities for development, inspection, testing, handling, and distribution of raw materials, components, in-process materials, and finished products. Examples include but are not limited to Batch records, logs, protocols, reports, etc.
ALCOA: A commonly used acronym for ‘Attributable, Legible, Contemporaneous, Original and Accurate.’
ALCOA+: A commonly used acronym for ‘Attributable, Legible, Contemporaneous, Original and Accurate’ which puts additional emphasis on the attributes being ‘Complete, Consistent, Enduring and Available’– qualities which are implicit in the basic ALCOA principles.
Accurate: Correct in all aspects or details. Accuracy is assured through equipment/instrument qualification, calibration and maintenance, validation, adherence to policies and procedures, data review, and self-inspection.
Audit trail: An audit trail is a form of metadata that contains information associated with actions that relate to the creation, modification, or deletion of GxP records. An audit trail provides for secure recording of lifecycle details such as creation, addition, deletion or alteration of information in an electronic record, without obscuring or overwriting the original record. An audit trail facilitates the reconstruction of the history of such events relating to the record regardless of its media, including the “who, what, when, and why” of the action.
Archival: Archiving is the process of protecting records from the possibility of being further altered or deleted and storing these records under the control of dedicated data management personnel throughout the required records retention period.
Attributable: Traceable to a unique individual. ‘Paper Record’ refers to the initials or hand-written signature of the individual, while ‘Electronic Record’ refers to the log-on user ID or electronic signature of the individual.
Principles of ALCOVA & ALCOVA+:
ALCOA stands for Attributable, Legible, Original, Contemporaneous, and Accurate. Afterwards, ALCOA was enhanced to ALCOA Plus (ALCOA+) by including a few more updates which are: available, enduring, consistent, and complete.
ALCOA guidelines:
Attributable- ‘Attributable’ means information is captured in the record such that it is uniquely identified as executed by the originator of the data (e.g., a person and/or a computer system). (The Person generating the data)
Legible- The terms ‘legible’, ‘traceable’, and ‘permanent’ refer to the requirements that data are readable and understandable and allow a clear picture of the sequencing of steps or events in the record. (Legible and Permanent).
Contemporaneous- ‘Contemporaneous’ is the process of documentation (on paper or electronically) at the time of the occurrence of an activity.
Original- ‘Original’ data includes the first capture or capture at the source of data or information and all subsequent data required to fully reconstruct the conduct of the GxP activity. (True Copy)
Accurate- ‘Accurate’ means that data are correct, truthful, valid, and reliable. ‘Complete’ means that all data from the analysis, including any data generated before a problem is observed, data generated after repeating part or all of the work, or re-analysis performed on the sample are contained in the data record. For hybrid systems, the paper output must be linked to the underlying electronic records used to produce it.
ALCOA+ guidelines:
Consistent- The data must be self-consistent.(‘Consistent’ means that all elements of the analysis, such as the sequence of events, follow-on, and data files are date-stamped (all processes) and timestamped(when using a hybrid or electronic system) in the expected order and such data are contained in the record.)
Enduring- Durable, Lasting throughout the data life cycle. ( ‘Enduring’ means that all data have been recorded on authorized media which can be preserved for a period, e.g., laboratory notebooks, numbered worksheets, for which there is accountability or electronic media. Data recorded on scrap paper or any other media that can be discarded later, e.g., backs of envelopes, laboratory coat sleeves Post‑It notes, etc. are not considered enduring.)
Complete-The data should be complete with all the records of changes, and reanalysis performed to the samples in the audit trail. The person reviewing the data should be able to reconstruct a complete picture. (The data must be whole; a complete set).
Available- Readly available for inspection or review purposes. (‘Available’ means that the complete collection of records can be accessed or retrieved for review and audit or inspection over the lifetime of the record.)
Important Concept:
Data: Data means all original records and certified true copies of original records, including source data and metadata and all subsequent transformations and reports of this data, which are recorded at the time of the GxP activity and allow complete reconstruction and evaluation of the GxP activity. Data may be contained in paper records (such as worksheets and logbooks), electronic records and audit trails, photographs, microfilm or microfiche, audio- or video files or any other media whereby information related to GxP activities is recorded.
Data Governance: The total of arrangements to ensure that data, irrespective of the format in which is generated, is recorded, processed, retained, and retrieved to ensure a complete, consistent and accurate record throughout the data lifecycle.
Electronic Records: Any combination of text, graphics, data, audio, pictorial or other information and/or representation in digital form that is created, modified, maintained, archived, retrieved, or distributed by a computer system.
GxP: Acronym for the group of good practice guides governing the preclinical, clinical, manufacturing, and post-market activities for regulated pharmaceuticals, biologics, medical devices, etc., such as good laboratory practices, good clinical practices, good manufacturing practices, and good distribution practices.
GMP Documents: All types of documents which have a direct or indirect impact on all aspects of the quality of drug substances/products and which are required to demonstrate or provide evidence of adherence to GMP standards and/or any other applicable regulatory requirements, are collectively referred as ‘GMP documents.’
Metadata: Metadata is data that describes the attributes of other data, and provides context and meaning. Typically, these are data that describe the structure, data elements, interrelationships, and other characteristics of data. It also permits data to be attributable to an individual.
Data Life cycle: All phases in the life of the data from generation and recording through processing (including analysis, transformation, or migration), use data retention, archive/retrieval, and destruction.)
True Copy: A true copy is a copy of an original recording of data that has been certified to confirm that it is an exact and complete copy that preserves the entire content and meaning of the original record, including in the case of electronic data, all metadata, and the original record format as appropriate.
Backup: A backup means a copy of one or more electronic files created as an alternative in case the original data or system is lost or becomes unusable.
Hybrid System/Approach: This refers to the use of a computerized system in which there is a combination of original electronic records and paper records which comprise the total record set that should be reviewed and retained. An example of a hybrid approach is where laboratory analysts us computerized instrument systems that create original electronic records and then print a summary of the results. The hybrid approach requires a secure link between all record types, including paper and electronic, throughout the retention period of the records. Where hybrid approaches are used, appropriate controls for electronic documents, such as templates, forms, and master documents, that may be printed, should be in effect.
Procedure or General Criteria:
1. Quality Assurance shall be responsible for the issuance, storage, retrieval, and destruction of controlled documents and records.
2. All documents should be designed, prepared, reviewed, and distributed as per the current version.
3. Ensure correct versions of records are used for data recording.
4. Documents should be approved, signed, and dated by the concerned authorized signatories.
5. All entries must be made only in a blue ball pen. Use of any other pen such as a roller ball or gel pen is prohibited. DO NOT use pencil, erasable ink, or correction fluid.
6. All entries must be legible and entered on the document at the time the function/activity is being performed. DO NOT document in advance, backdate, or pre-date any information.
7. All entries must be signed/initiated and dated properly. Specific requirements are noted in each document. Staff are not permitted to sign for another member of staff unless delegated.
8. No entry shall be overwritten.
9. All master documents shall have an effective date, approval date, and current version number.
10. Handwritten signatures must be unique to the individual and listed within the Specimen Signature Register to ensure that the signature is traceable to the concerned employee (or contractor). This register shall have a list of personnel with long and short specimen signatures (initials).
11. The meaning of a signature shall be communicated to the personnel involved in signing off GMP documents.
12. A single strike-out line must be used always to mark the incorrect entry in such a manner that the original entry remains readable. The correct entry shall be written near to the strikeout entry. The reason for alteration, e.g., transcription error, typographical error, recording error, calculation error, etc., shall be recorded. Wherever necessary, detailed reason/justification for such corrections shall be provided for better clarity.For example, in case ‘01/01/15’ is recorded by mistake in place of ‘01/01/16’ and this the mistake is observed later during the BMR review on 04/01/16, it can be corrected at a later date in the following manner:
01/01/15 Sign/Date
01/01/16 (Reason)
If changes/corrections have been made to critical data entries, it must be verified that a valid reason for the change has been recorded and supporting evidence for the change is available.
13. If the information asked for in a particular instruction/column does not apply to the activity performed, the words ‘NA’ should be written against such instruction. If the information is asked for in case of a complete section or complete table or complete page then ‘NA’ shall be written against such instruction, a diagonal line shall be drawn across the section or table or page, and shall be initialed with date.
14. If an option is provided in the form, the applicable option shall be selected by crossing out the non-applicable option for e.g. Required/Not required. If the option is to be indicated in the boxes provided in the document, the applicability shall be indicated by putting a tick mark (√) in the appropriate box
15. If a decimal value is a fraction of 1 then a zero must be placed before the decimal point. Example: record 0.95 rather than .95
16. Electronic data shall not be destroyed. All electronic data shall be perpetual.
In addition to providing a tool to ensure that the distribution system is protected from counterfeit goods, unapproved imports, illegal drugs, stolen goods, subpar goods, adulterated goods, and/or misbranded pharmaceutical products, quality assurance should guarantee that the quality of a pharmaceutical product is maintained through acceptable controls of the various activities that occur during the distribution process.
Get ready to use editable documents in MS Word Format (Regulatory standard SOPs) Only Rs-49₹/-
Contact:
Email: contact@likeways.co.in
WhatsApp/Telegram No: 9738137533 (Only for Message)
Note: Kindly note that you will receive the document through email only after the payment has been completed. Please send the screenshot of your payment confirmation via WhatsApp/Telegram. A payment QR code will be provided from our end.
